TrakSYS for Life Sciences
Just What the Doctor Ordered

Trusted by Leading Pharmaceutical, Biotech, and Medical Manufacturers Worldwide
TrakSYS MES software has been deployed at thousands of factories in over 100 countries, including many leading pharma and life sciences manufacturers.








The Prescription for a More Productive and Profitable Operation
As a life sciences manufacturer — including pharmaceuticals, biotechnology, and medical devices — you operate in a high-volume environment where quality is paramount. After all, the value of your brand is tied closely to the quality of just one or several products. In addition, you must conform to strict regulations, such as US FDA 21 CFR Part 11 and European Annex 11, and follow current Good Manufacturing Practices (cGMP). You’re focused on optimizing operations, to increase quality and productivity, and add a few more points to the bottom line. TrakSYS can help.
Improve Total Product Quality
Reduce Production Costs
Ensure Complete Batch Safety

Comply With Regulations
How TrakSYS Helps You Gain Greater Visibility, Knowledge, and Control
TrakSYS MES software helps pharmaceutical and other life sciences manufacturers enable operational excellence initiatives, meet safety compliance requirements, and support sustainability efforts. Here’s how:
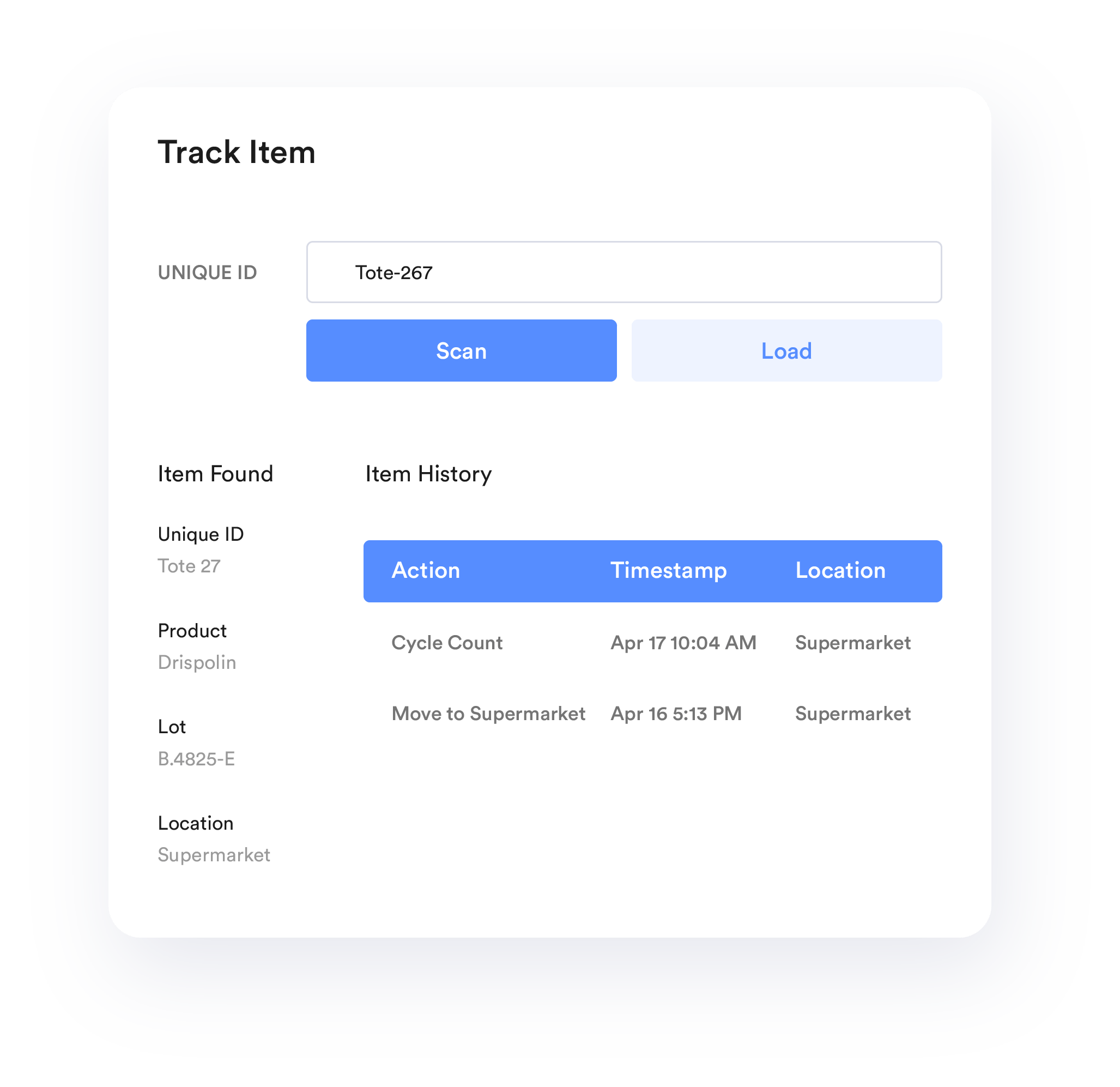
Batch Recipe Management
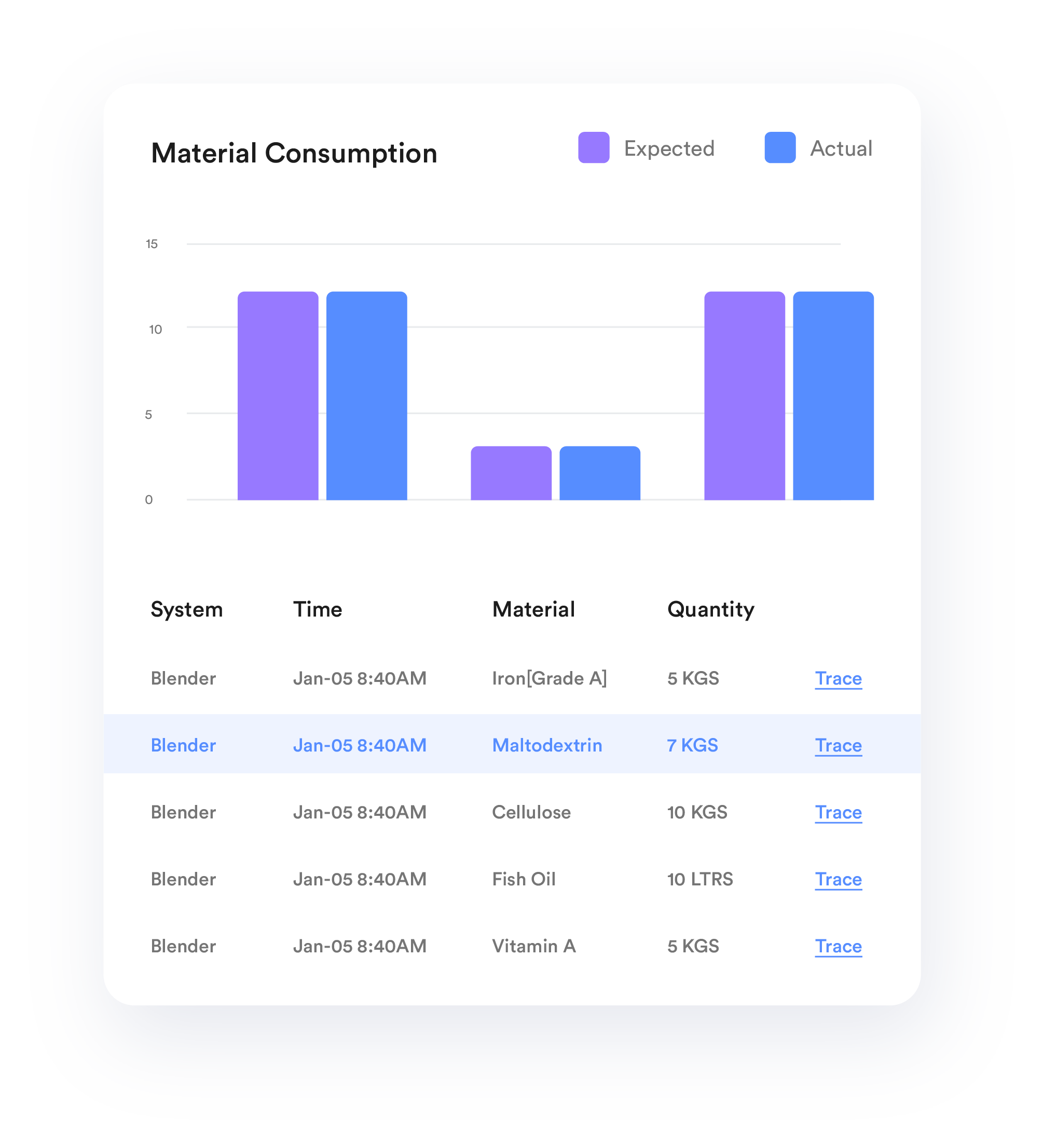
Integrate TrakSYS with automated material dispensing systems to ensure each batch is comprised of the appropriate amount of a given ingredient.
Electronic Records
TrakSYS offers comprehensive electronic records solutions, including Electronic Batch Records (EBRs) and Electronic Device History Records (eDHRs), designed to help pharmaceutical, life sciences, and medical device companies work more efficiently. Whether you’re managing batch processes or discrete manufacturing, TrakSYS automates critical record-keeping tasks—capturing changes, actions, recorded values, and electronic signatures—to create a complete, compliant audit trail at every stage of production.
Ensure a clear audit trail for all actions within the manufacturing process.
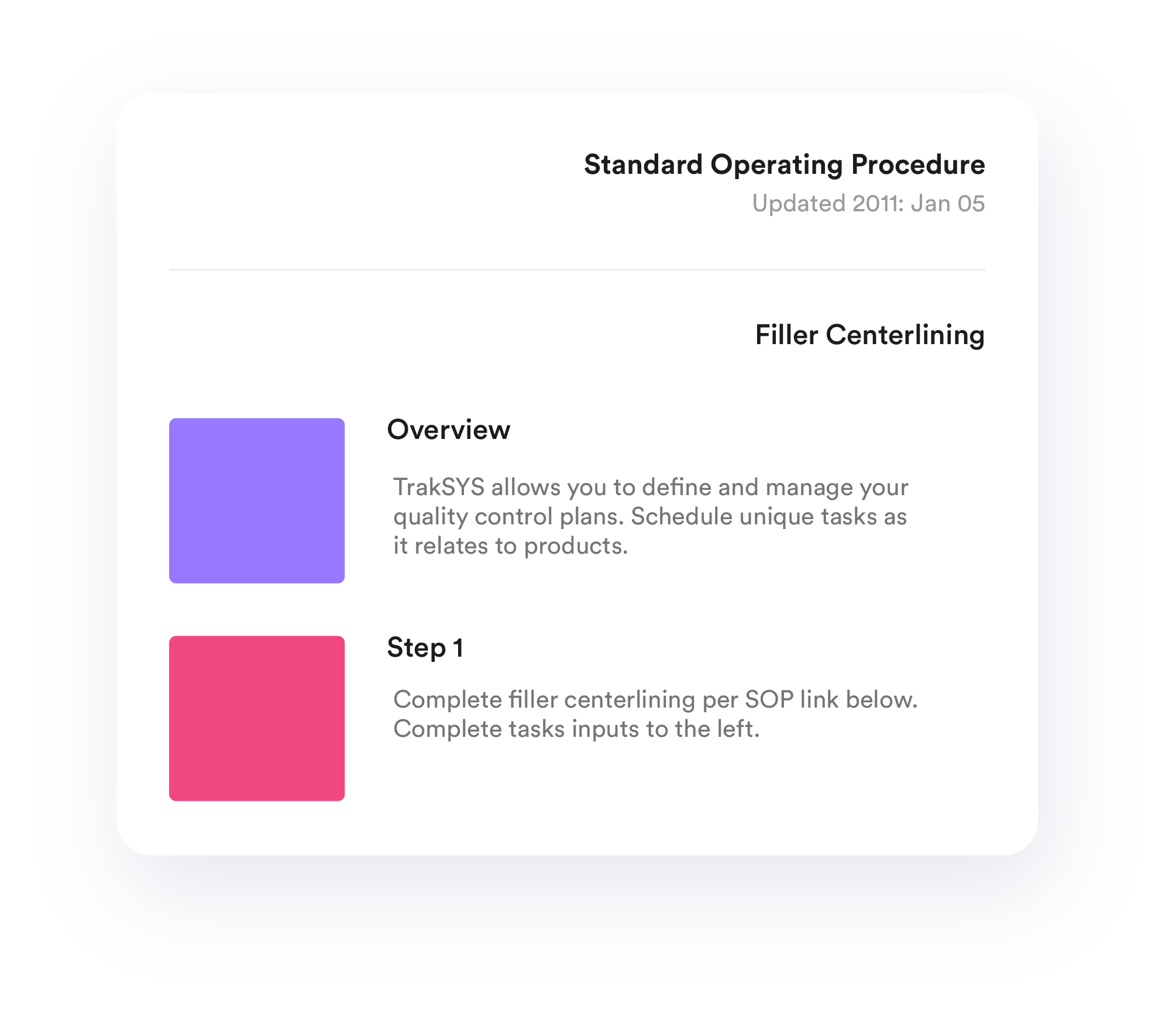
SOP Implementation
Capture accurate, real-time production data by integrating TrakSYS into existing systems or by using TrakSYS smart devices.
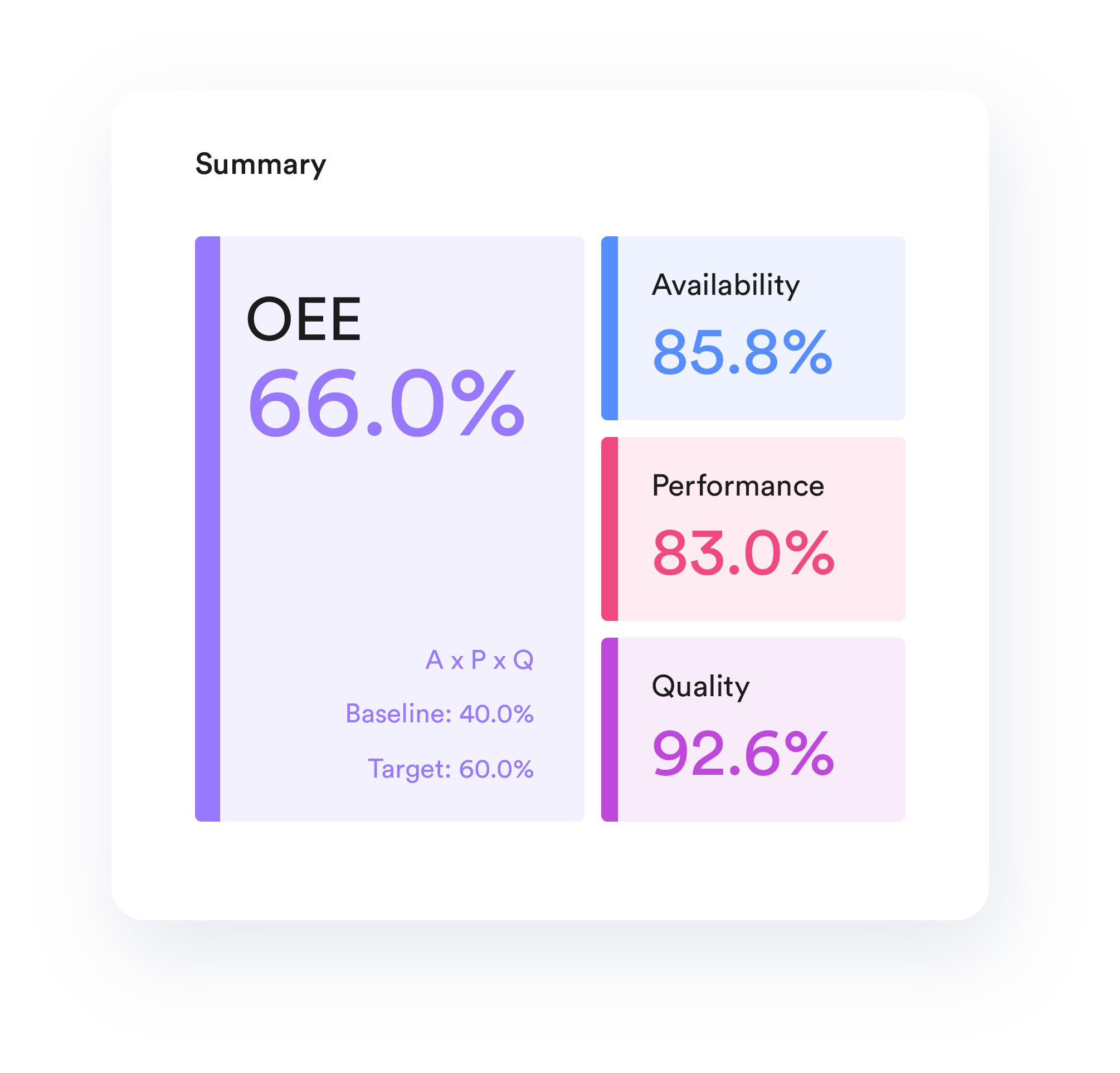
Increased Productivity
Life Sciences Related Content


What are You Working on Right Now?
Performance? Quality? E-Records? Tell us what you’re working on.
Chances are, we can help.
