Streamline Compliance, Improve Quality
Today’s Regulatory Environment Demands Better Quality and Compliance Processes
For those in industries regulated by the FDA and EU compliance, it is crucial to have systems that assure stringent compliance with government and industry standards — as well as extensibility to comply with future requirements. TrakSYS is a unified Manufacturing Operations Management (MOM) platform that helps manufacturers meet an assortment of regulatory requirements and standards including FDA’s 21 CFR Part 11, Part 820, GMP, ISO 13485, European Annex 11, and ISO 9001.

Comply with Regulatory Requirements

Protect Customer Safety and Brand Reputation
Improve Efficiency and Accountability

Minimize Administration Costs
TrakSYS makes it possible to easily define the rules of automating your data collection throughout the production process. With digital record-keeping, ensuring Traceability and Compliance are built-in by design. All necessary documents are easily accessible to authorized users through a web interface. Interactive reports and dashboards provide real-time notification of non-conformance, contextualized information for identifying root causes, and comprehensive audit trail.
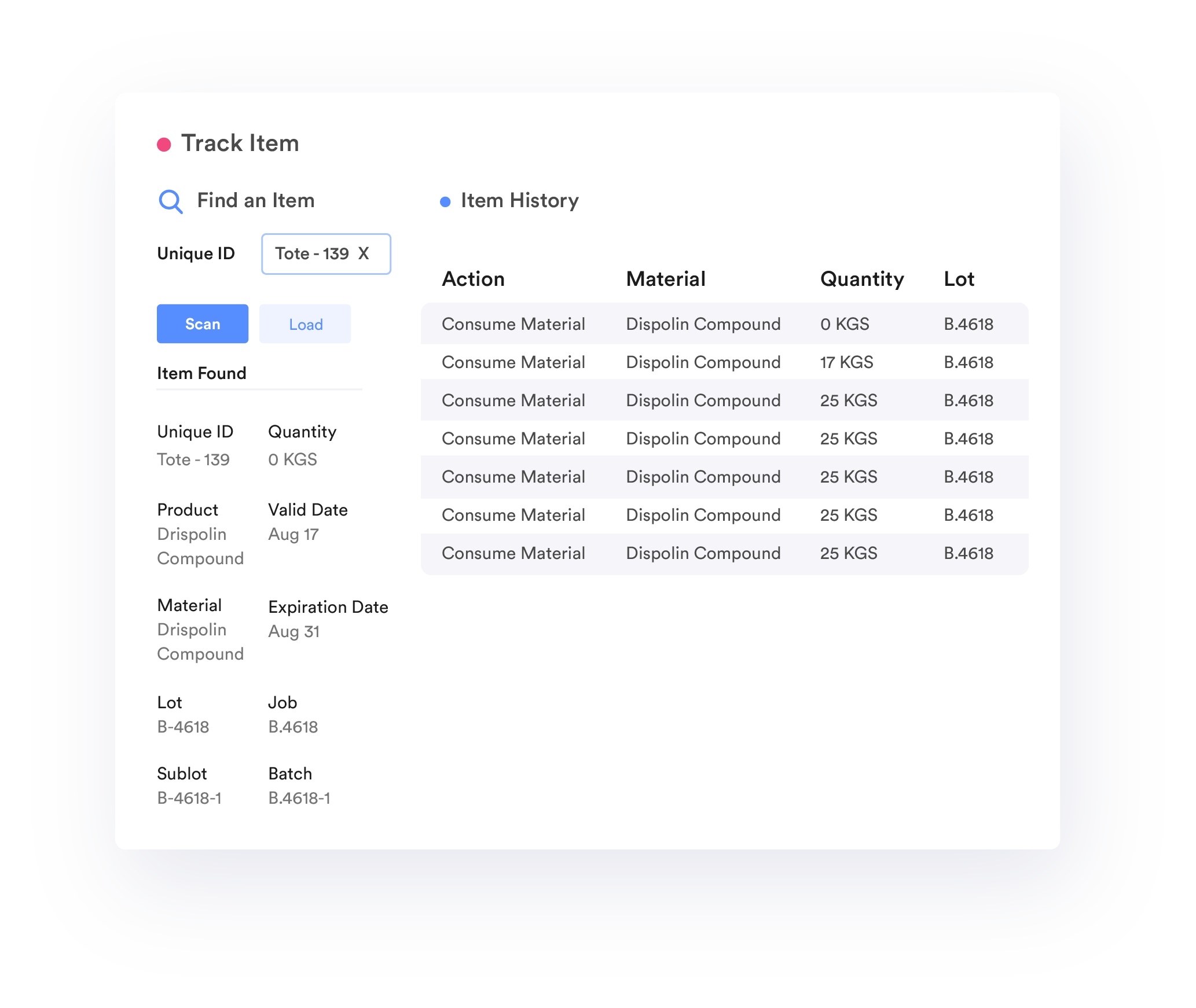
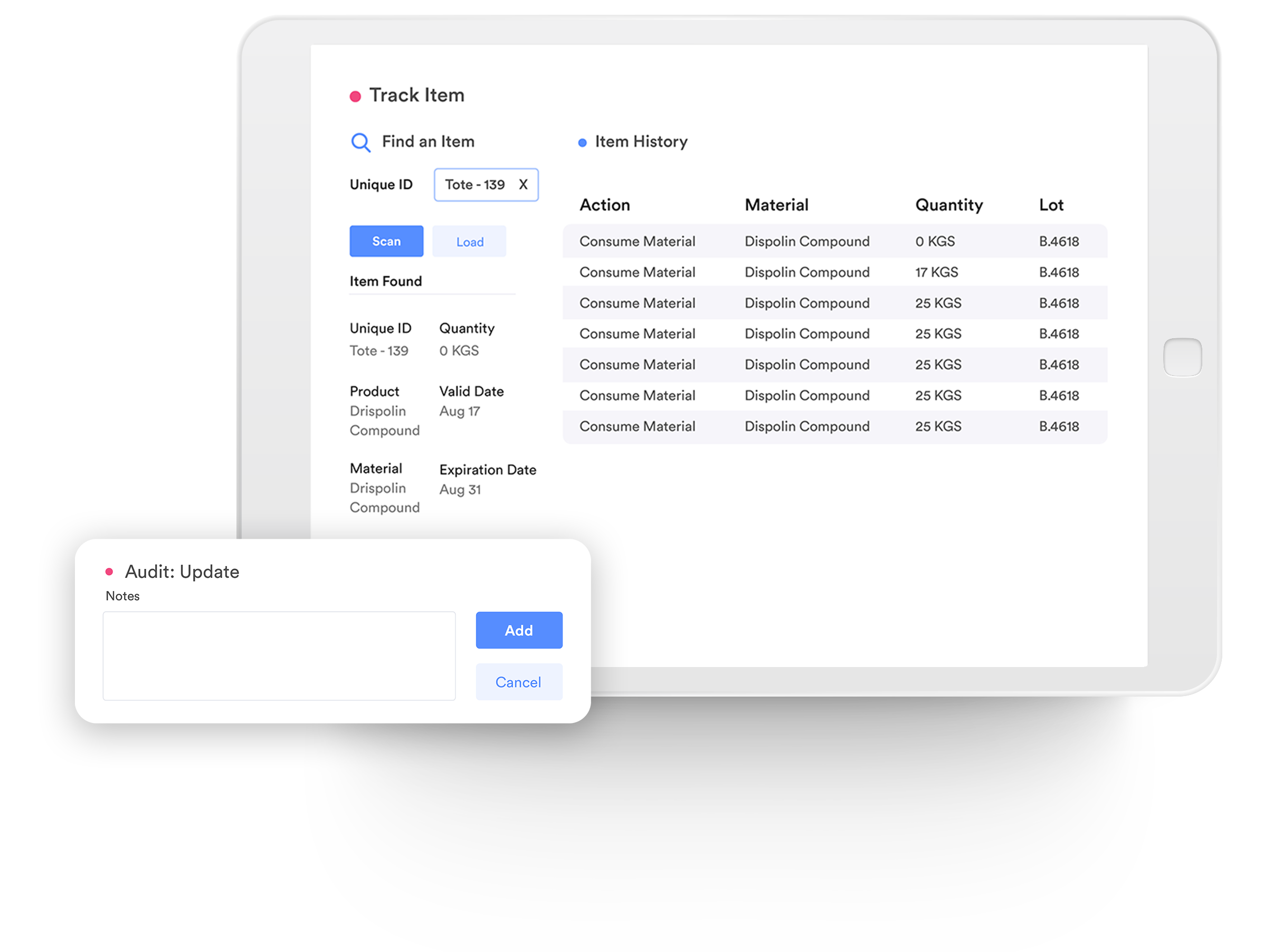
Traceability
Record, track, and trace the genealogy of raw materials, Work-in-Process (WIP), and go-to-market products. Provide real-time transparency, to help you proactively limit the impact of any quality problems before you ship the product.
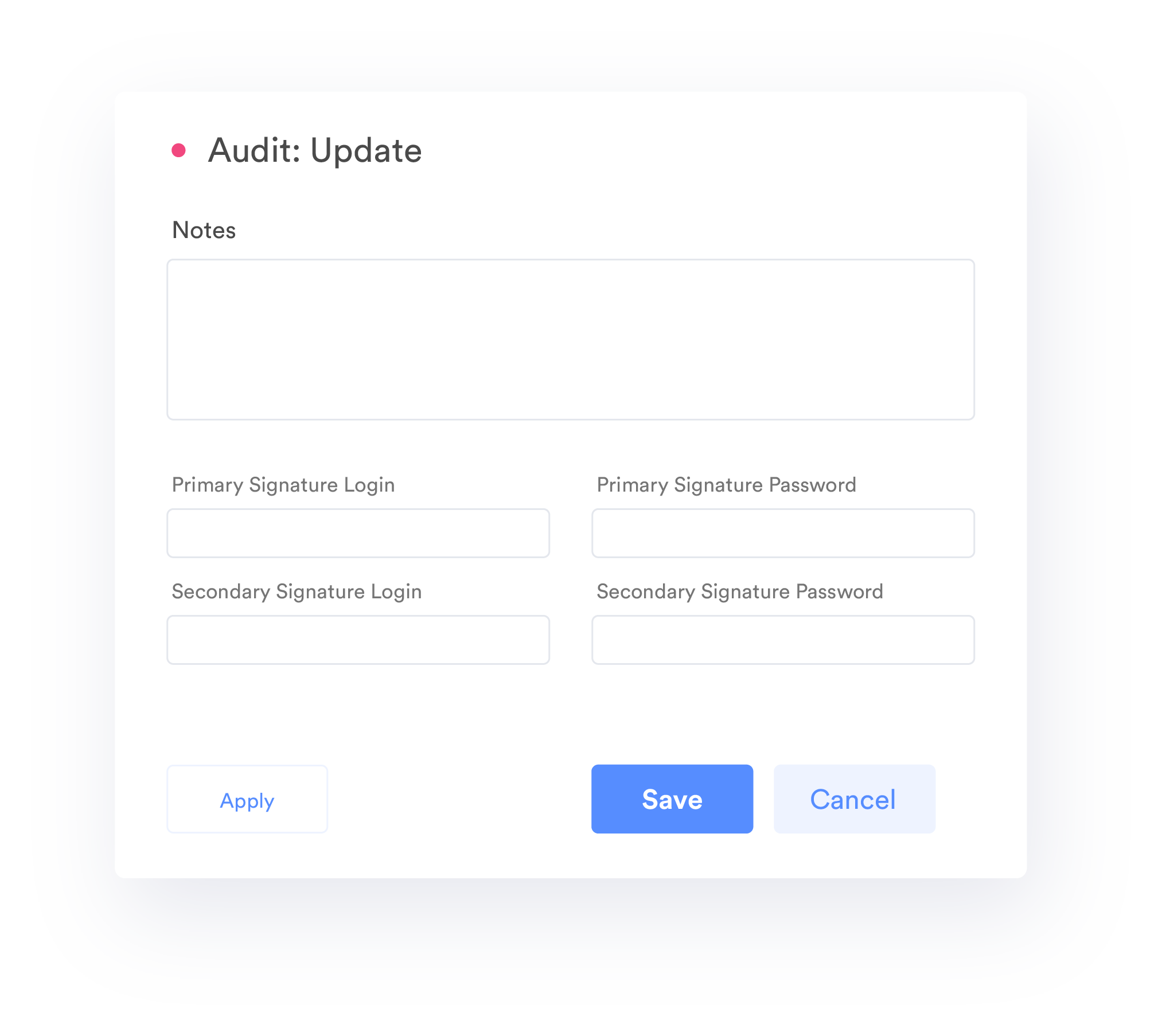
21 CFR Compliance-Digital Signature
Automate comprehensive record collection with electronic signatures while maintaining 21 CFR Part 11 and Part 820 compliance.
Change Management
Managing change is a critical element of a risk mitigation strategy. A failure to manage change is a risk in and of itself that commonly traces back to major crises. TrakSYS ensures controls are in place to introduce system changes as well as maintain data integrity.
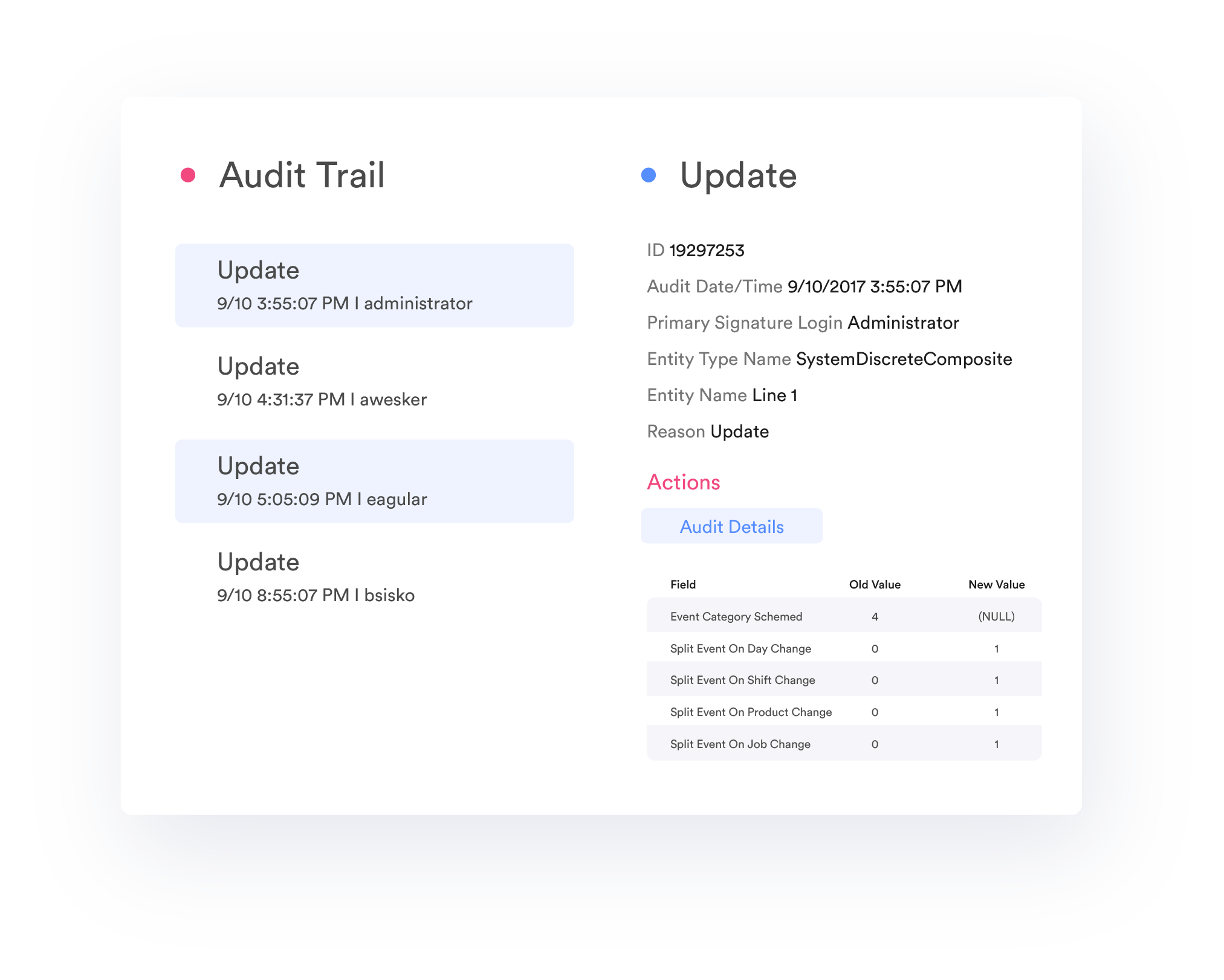
Audit Trail
TrakSYS allows configuration of user and group roles to maintain data integrity. As well as a configurable tracking of changes to system configuration, actions, data records, and electronic signatures to create a comprehensive audit trail.
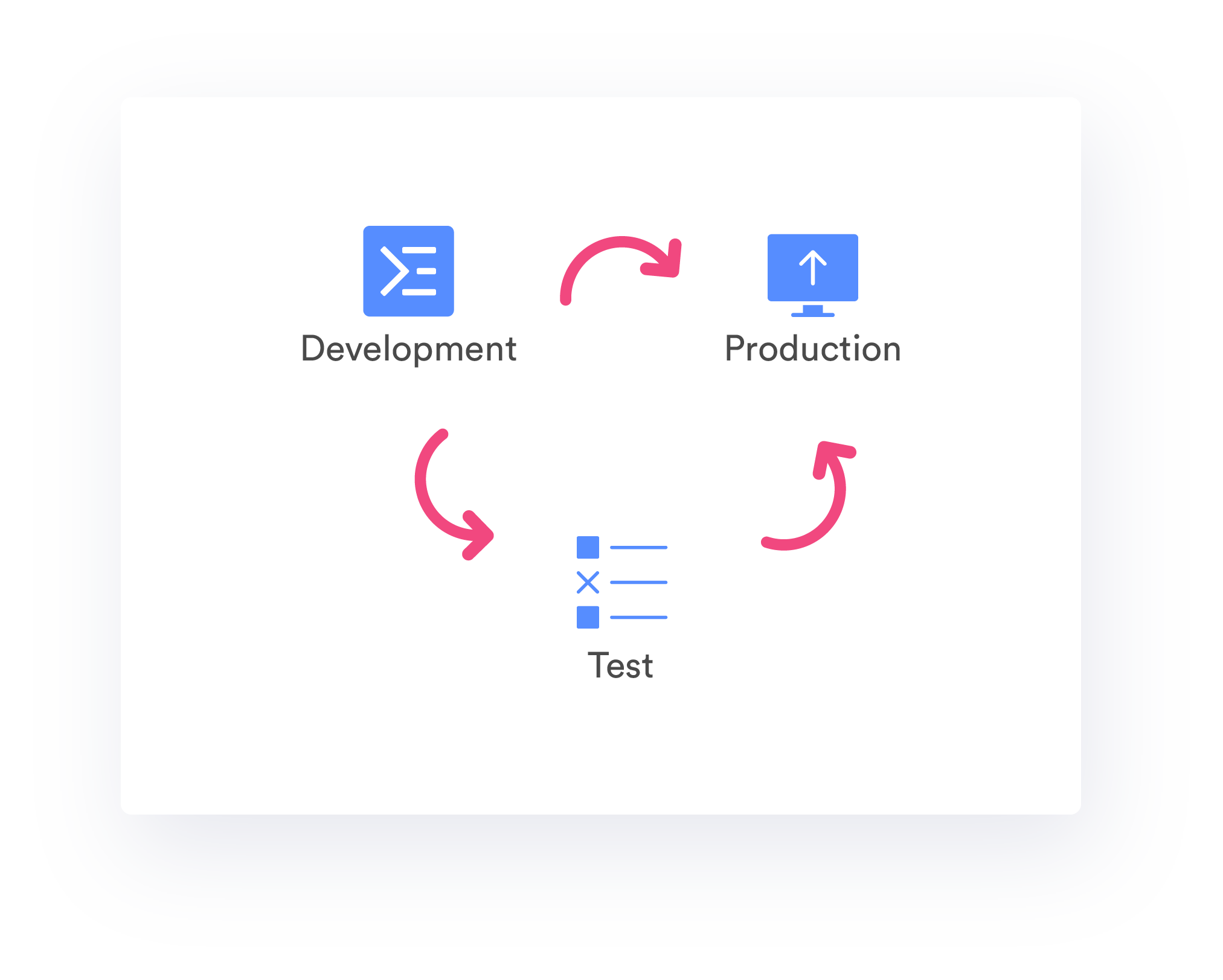
Development-Test-Production Environments
TrakSYS architecture allows for parallel environments to manage FDA validated software installations. Develop without interruption, while system changes are tested and validated for production activation.
Configurability
Web-based configuration tools and an integrated scripting environment allow the construction of tailored batch management solutions without the need for custom code.
Extensibility
TrakSYS is a full MES software platform, with standard function libraries that can be readily adjusted to create focused batch management solutions in a fraction of the time compared to traditional development.
